About ALZ-NET
Nearly seven million Americans are living with Alzheimer’s disease or a related dementia. However, with new FDA-approved treatments that can change disease progression, coupled with more than 140 therapies in clinical trials or under regulatory review, we are in a new era of treatment.
To better understand how new and future FDA-approved Alzheimer’s therapies work in real-world settings, the Alzheimer’s Association convened a group of clinicians and research experts in 2021 to conceptualize a first-of-its-kind network dedicated to tracking real-world diagnostic and treatment outcomes. In August 2022, the Alzheimer’s Network for Treatment and Diagnostics (ALZ-NET) was launched.
ALZ-NET is a voluntary provider-enrolled patient network that collects real-world clinical and imaging data from people evaluated for or treated with new FDA-approved Alzheimer’s therapies, including treatments designed to slow disease progression and those that treat cognitive, behavioral, or neuropsychiatric symptoms of Alzheimer’s. ALZ-NET is a resource for evidence gathering, information sharing, and education across clinical and research communities, encouraging innovative, inclusive research and supporting opportunities to improve care. ALZ-NET is sponsored by the Alzheimer’s Association and is managed and operated by the American College of Radiology.
Our approach
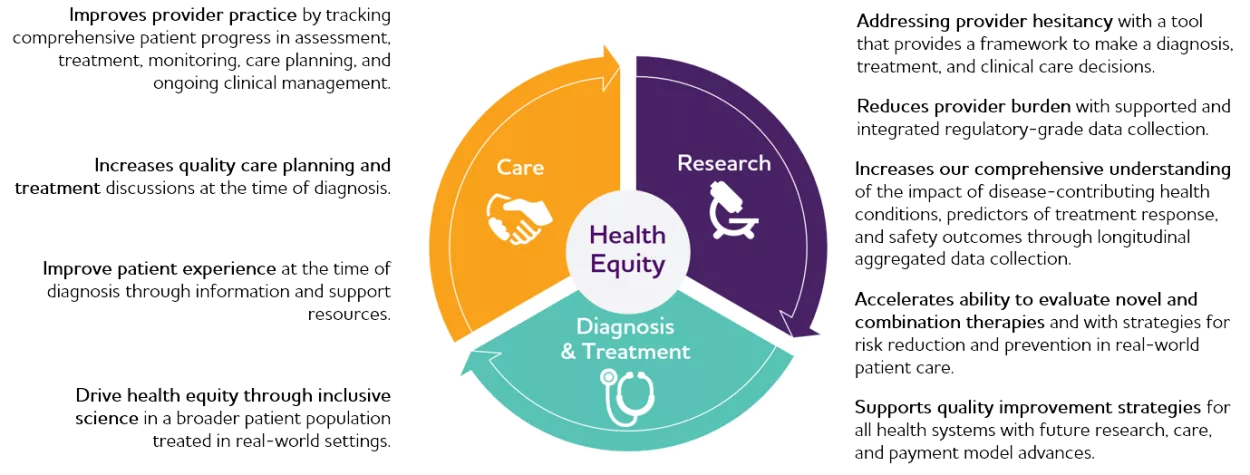
ALZ-NET will be an integrated network benefiting all communities.
ALZ-NET will address the urgent need for a cohesive approach to evidence-based detection, diagnosis, treatment, and quality care for all individuals and their families affected by Alzheimer’s disease as we enter a new phase of treatment. ALZ-NET also seeks to reduce the burden of clinical assessment, monitoring, and care planning while streamlining real-world data collection for research.
ALZ-NET will collect and assess real-world data, such as information collected during office visits, patient medical records, and, in the future, through electronic health records and other sources. It will collect and archive longitudinal patient data, including demographic, medical, neurologic, cognitive, functional, genetic, diagnostic, imaging, biomarker, treatment, and safety data.
The network will collect high-quality data that reflects how people function and receive real-world care, which compliments what we can learn from traditional clinical trials. ALZ-NET will also enhance clinical trial and study recruitment through its inclusive approach to generating a broad provider and patient network. It is currently used to evaluate novel therapies and will inform future combination approaches and risk reduction and prevention strategies.
ALZ-NET will enable responsible sharing of de-identified data, brain imaging, and biospecimens with the research community and other partners. ALZ-NET plans to make its data publicly accessible on an ongoing basis throughout the duration of the project.
The network fosters a collaboration across sectors to improve care and support innovative research by connecting health care providers, researchers, life sciences and health information technology companies, payors, public health entities, government and regulatory agencies, policymakers, and private and public funders.
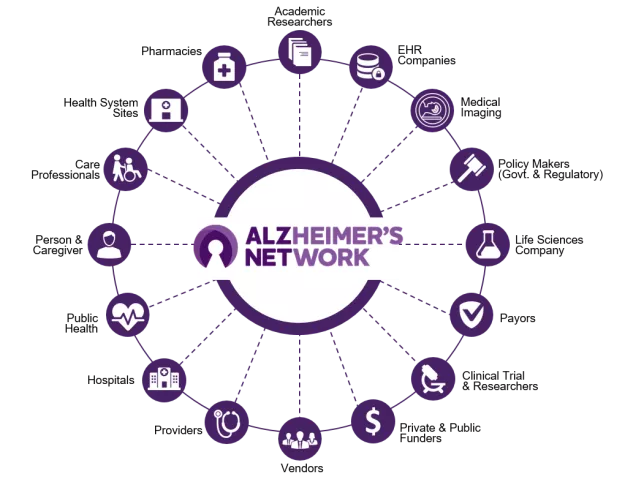
Enrollment in ALZ-NET
ALZ-NET is enrolling clinical sites across the country. If eligible, the provider at the site will encourage and seek the consent of patients to participate in ALZ-NET data collection. Health care providers participating in ALZ-NET are responsible for submitting their patients’ data and information to the network. Find a health system or health care provider participating in ALZ-NET in your state or near your ZIP code.
Participation in ALZ-NET by health care providers and patients is encouraged by the FDA in the prescribing information of approved Alzheimer’s medications and in the medical policies of private payers. ALZ-NET is approved by the Centers for Medicare and Medicaid Services (CMS) as a Coverage with Evidence Development (CED) study. It can be used as a pathway to Medicare coverage for anti-amyloid Alzheimer's therapies that have received traditional (full) FDA approval.
Questions ALZ-NET may answer
- What is the long term effectiveness of a treatment?
- What are long term safety considerations?
- Who is most likely to respond well and benefit from different treatments?
- Who is most likely to have treatment-related side effects?
- How do new treatments interact with other medications people are taking?
- What happens when the available treatments target different aspects of the disease?
- Which treatments might work well together as combination therapies?
- And much more.